Peter Teska: Global Subject Matter Expert
Rebecca Battjes: Senior Clinical Advisor for Infection Prevention
Emerging pathogens are microorganisms that cause disease in a population for the first time or pathogens that previously existed, but suddenly have shown an increase in geography or disease incidence (WHO, 2014) (Weber, 2019). For common pathogenic (or disease-causing) microorganisms, the accepted methods for addressing infection risk are generally well understood. Typically, infection prevention and control recommendations have been standardized by public health organizations, such as the World Health Organization (WHO) or the Centers for Disease Control and Prevention (CDC).
For emerging pathogens, the epidemiology of the associated disease may be less well understood, and evidence-based guidelines are not always readily available. As a result, there may be more variability in how public health and healthcare facilities work to address the risk. On any given day, a patient may visit a healthcare facility, infected with a rare pathogen. This is increasingly likely, given wide-spread global travel. Understanding how to both treat the patient and protect the healthcare workers and other patients in the facility are critical for building and maintaining the public's trust in the healthcare system.
The recent COVID-19 pandemic is a relevant example of how a lack of scientific evidence led to ever-evolving recommendations, and ultimately, confusion and distrust among both the general public and healthcare professionals. Many people have now forgotten that in the earliest stages of the COVID-19 pandemic, laboratory testing was largely unavailable for healthcare facilities, let alone the general public. In the spring of 2020, physicians did not know what medications would improve or worsen the viral infection. Likewise, the effectiveness of non-pharmaceutical interventions (NPIs), such as masking, including the use of particulate respirators, was not fully understood. When evidence demonstrated that persons presumably without any active symptoms could be contagious, the situation became significantly more complex. Everyone became a potential spreader. Even as more sophisticated diagnostic tests became available (e.g., polymerase chain reaction [PCR] and antigenic testing), the window of contagiousness was still not well understood. Healthcare professionals were at a high risk of infection during the first stages of the COVID-19 pandemic (Baker, 2020) at least in part due to a global gap in understanding the epidemiology of SARS-CoV-2.
In healthcare facilities, such as hospitals and nursing homes, addressing emerging pathogen infection risk presents substantial challenges. Many patients and residents have complex medical needs, including chronic illnesses and compromised immune systems. Prolonged exposure to acute and long term healthcare facilities is also an inherent risk, as there are many opportunities for infection transmission. One of the greatest challenges of a previously unknown or emerging pathogen is the initial inability to identify it using existing laboratory methods, as was an issue at the start of the COVID-19 pandemic.
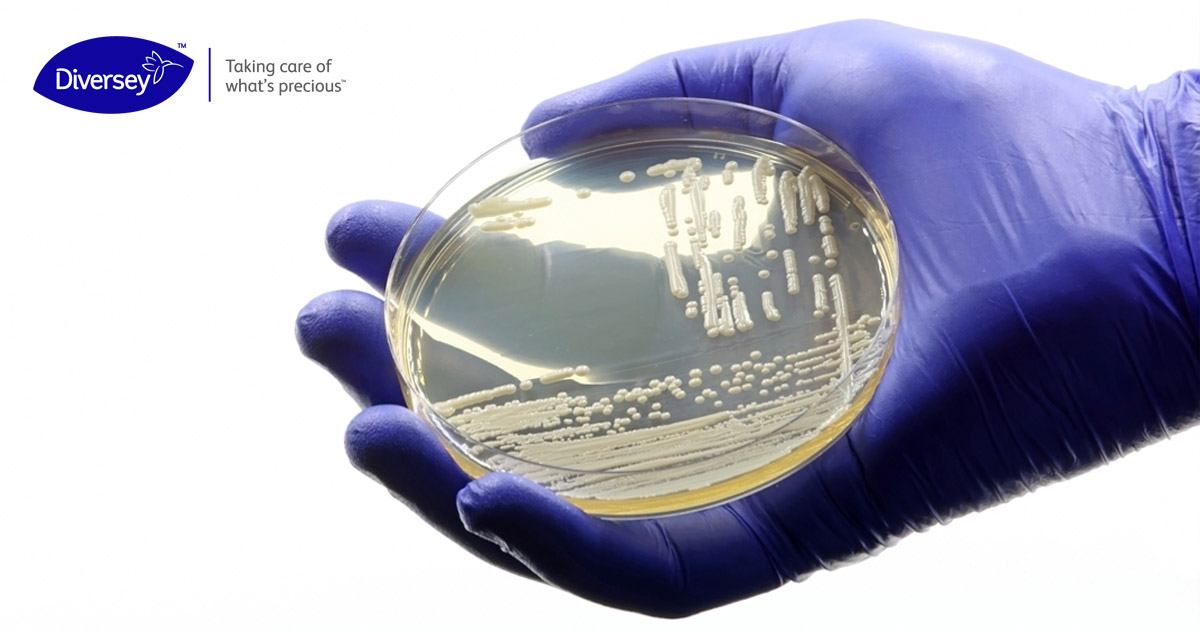
One such emerging pathogen is the yeast, Candida auris (Weber, 2019) (CDC, 2020). First identified in the ear canal of a patient in a Japanese hospital in 2009, C. auris is a novel Candida species that has spread to more than 50 countries with many countries reporting rapidly increasing numbers of cases (WHO, 2022). C. auris has a high potential to cause outbreaks because it is environmentally stable and readily grows biofilms in the environment. Biofilms are protective environments that many microorganisms create to bind together and survive on surfaces. C. auris can survive in biofilms at least one to four weeks on hard and soft surfaces (Weber 2019). On such surfaces, C. auris is resistant to many commonly-used disinfectants and moderately resistant to UV-C light. It can be easily misidentified during routine laboratory testing, particularly in hospital laboratories without access to more sophisticated equipment. Because C. auris is relatively new, the effectiveness of the various recommended preventative measures are not well-established, (WHO, 2022) (CDC, 2020) (CDC, 2023). There are no vaccines for C. auris, and it is inherently resistant to most antifungal drugs. If the disease course was mild, this could be less concerning, but the mortality rate for C. auris ranges from 29 ? 53% in some studies (WHO, 2022).
While researchers are quickly moving to fill data gaps, recommendations to stop the spread of C. auris in healthcare facilities have been developed (CDC, 2023). Current CDC (2023) recommendations for healthcare facilities include the following:
For Environmental Services (EVS) professionals, adhering to existing policies and procedures is paramount. Once C. auris enters a facility, it can be found in the rooms of patients and residents both with and without known infection or colonisation (Kumar 2019, Sexton 2021). EVS compliance with transmission-based precautions, including proper use of personal protective equipment (PPE), hand hygiene and disinfection of reusable cleaning tools is as important as the clinical team's compliance. In outbreak situations, increased disinfection of high-touch surfaces (e.g., twice daily or more) in occupied rooms may be considered. More frequent disinfection, however, requires more EVS staffing.
For Infection Preventionists, proper implementation of the CDC and WHO recommendations are expected to provide adequate control of the spread of C. auris within a healthcare facility. Concerns about how easily C. auris can spread drive the focus on these preventative measures. However, it is not clear which of these recommended practices are likely to have the biggest impact on controlling infections and thus should be prioritized in a healthcare facility. Ensuring disinfectants with proven efficacy against C. auris used by both the clinical and EVS teams is an excellent place to start.
Multiple studies have shown that a number of commonly used disinfectants demonstrate reduced efficacy against C. auris compared to reference organisms, such as Candida albicans. As a result the use of a disinfectant with demonstrated efficacy against C. auris is recommended (CDC, 2023) (Weber, 2019). One such study (Rutala, 2019) showed a quaternary ammonium chloride (i.e., ?quat?) disinfectant achieving only 1.7 log10 of efficacy while other disinfectants using different actives demonstrated > 4 log10 of efficacy. This is particularly relevant in countries and regions who continue to use quats as their primary disinfectant.
Because it can readily grow environmental biofilms, C. auris is more resistant to standard cleaning and disinfection procedures. Standard efficacy tests used for registration in the US and EU require testing the vegetative form of the yeast rather than the yeast in a biofilm. Because of these significant differences it is likely that some products marketed with claims against C. auris are actually less efficacious if they lack the ability to penetrate environmental biofilms.
The rise of C. auris demonstrates the importance of rapid investigation when an emerging pathogen first appears so that healthcare facilities and public health authorities can provide evidence- based recommendations to prevent transmission. Regulatory agencies, too, should respond to advances in scientific research and be prepared to update testing protocols accordingly. Ultimately, despite the ongoing threat of emerging pathogens, the fundamentals of infection prevention and control stand the test of time. As we approach International Infection Prevention Week beginning October 15th, 2023, the Association for Professionals in Infection Control & Epidemiology's (APIC) theme focus on those six pillars: cleaning and disinfection, hand hygiene, vaccination, PPE, injection safety and respiratory etiquette.